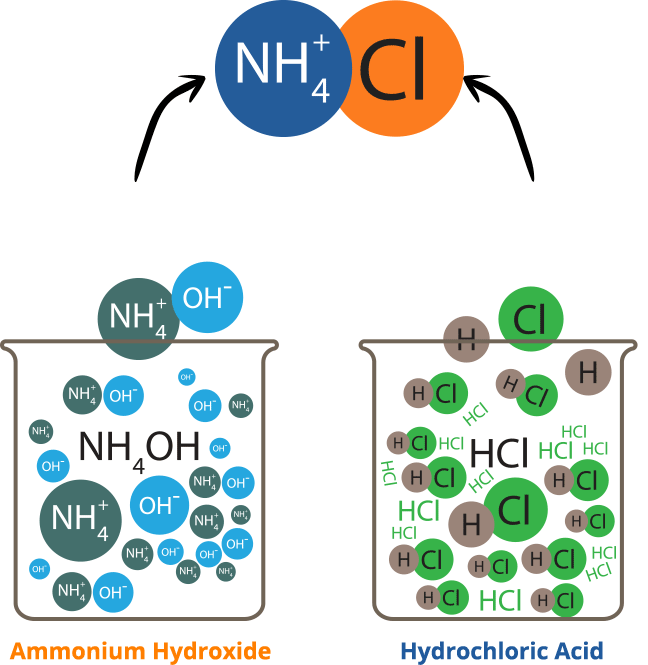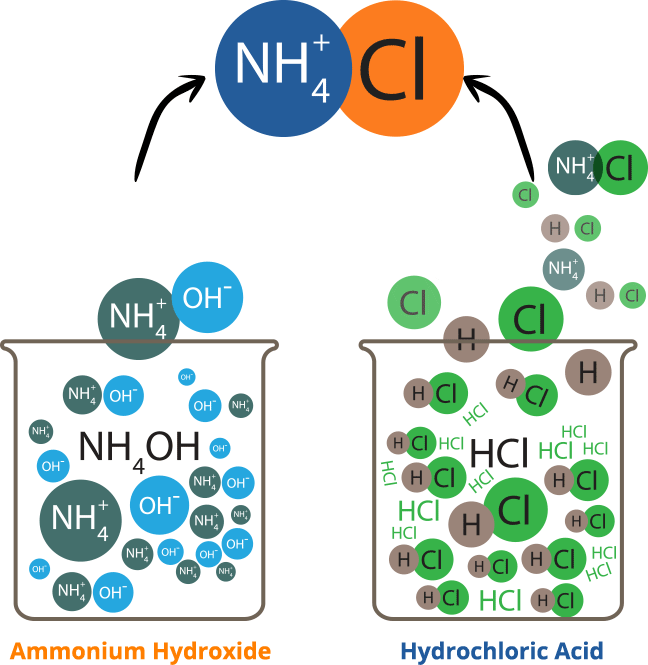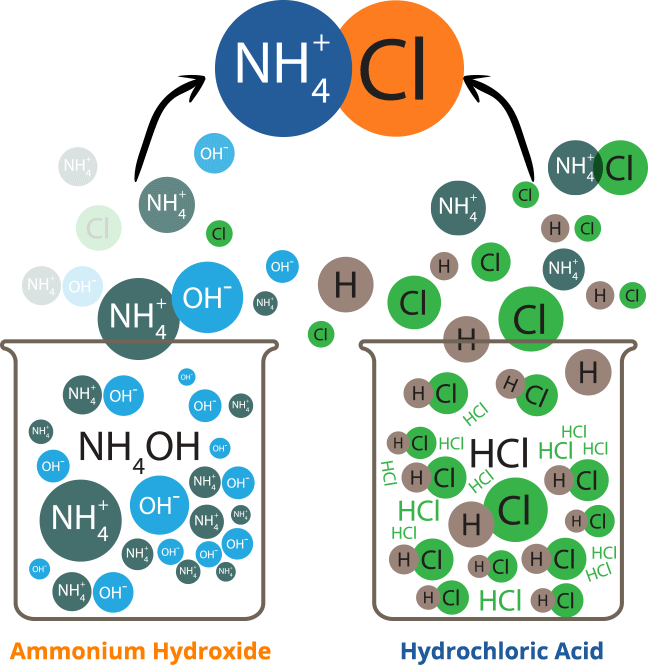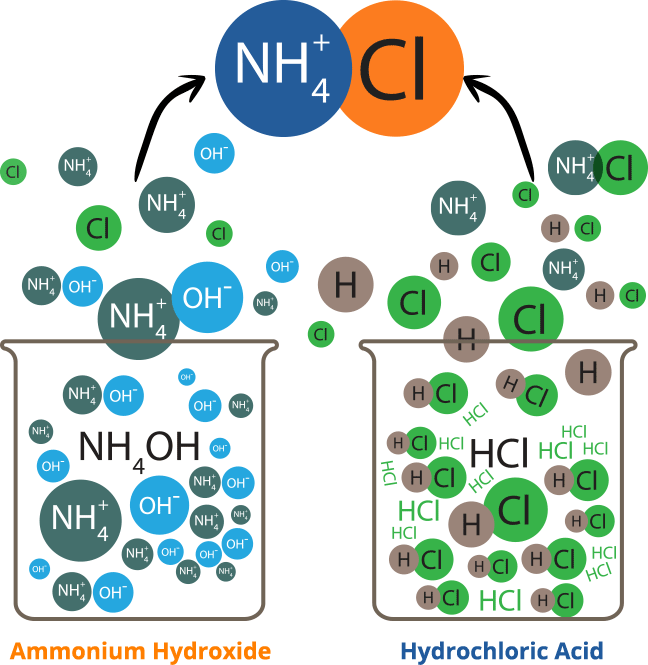
Ammonium chloride is an inorganic chemical compound, with the formula nh4cl. It is a white or colourless, odourless, cubic crystalline salt with a biting taste. Soluble in: ammonia, water, methanol, alcohol, glycerol and hydrazine Solutions of ammonium chloride are mildly acidic. It is prepared commercially by reacting ammonia, nh3, with hydrogen chloride, hcl.
FORMULA: NH4CL
MOLAR MASS: 53.491 G/MOLE
SUBLIMATION POINT: 338 °C
DENSITY: 1.53 G/CM³
| Characteristics | Range |
|---|---|
| Dry Basis Assay (NH4Cl) | 98.50% min |
| Moisture | 5% max. |
| Iron | 200ppm max. |
| Sulphated Ash | 0.3% max. |
| Residue on Ignition | 0.3% max |
| pH of 5% Solution | 4.5To5.5 |
| Matter insoluble in water | 0.3% max. |
| Heavy Metals as Pb | 5 ppm max |
| Arsenic | 5 ppm max |
Ammonium chloride is used as a catalyst in many reactions. It is also used in the production of many api, pharmaceuticals intermediates, synthetic organic dyes, pigments and other organic and ionorganic chemical products.
In wood working, a solution of ammonium chloride and water, when applied to unfinished wood, will burn when subjected to a heat gun resulting in a branding iron mark without use of a branding iron. The solution can be painted onto the wood or applied with a common rubber stamp.
Ammonium chloride is used as an expectorant in cough medicine. Its expectorant action is caused by irritative action on the bronchial mucosa, which causes the production of excess respiratory tract fluid, which presumably is easier to cough up.
Ammonium chloride is used as a systemic acidifying agent in treatment of severe metabolic alkalosis, in oral acid loading test to diagnose distal renal tubular acidosis, to maintain the urine at an acid ph in the treatment of some urinary-tract disorders.
Ammonium chloride, under the name sal ammoniac or salmiak is used as food additive, working as a yeast nutrient in bread making. It is a feed supplement for cattle. Ammonium chloride is used to spice up dark sweets called salmiak (popular in nordic and other nearby countries), in baking to give cookies a very crisp texture, and in the liquor salmiak koskenkorva for flavouring. In iran, tajikistan, india, pakistan and arab countries it is called "Noshader" and is used to improve the crispness of snacks such as samosas and jalebi.
Ammonium chloride has been used historically to produce low temperatures in cooling baths. Ammonium chloride solutions with ammonia are used as buffer solutions including ack (ammonium-chloride-potassium) lysis buffer.
Ammonium chloride is used in a ~5% aqueous solution to work on oil wells with clay swelling problems.
Ammonium chloride is used as an electrolyte in dry cell batteries. A standard dry cell comprises a zinc anode, usually in the form of a cylindrical pot, with a carbon cathode in the form of a central rod. The electrolyte is ammonium chloride in the form of a paste next to the zinc anode. The remaining space between the electrolyte and carbon cathode is taken up by a second paste consisting of ammonium chloride and manganese dioxide, the latter acting as a depolarizer.
In cosmetics and personal care products it is use in the formulation of bath products, soaps and detergents, cleansing products, hair coloring products and other hair care products including shampoos and hair conditioners. Ammonium chloride is used as a viscosity increasing agent. (substances that increase the thickness of the aqueous (water) portion of cosmetic products.)
In the glue that bonds plywood.
Ammonium chloride is most commonly applied as a hardener of uf resin. It is an affordable and relatively inexpensive reagent.
Ammonium chloride is used in the textile industries in dyeing printing and cotton clustering.
Ammonium chloride is used in leather during the deliming process and helps remove lime from the hides or skins to make the leather more soft and easy for tanning.
In galvanizing steel to be galvanized passes through an acidic cleaning process to remove iron oxide "Mill scale". After this process, the surface of the steel is very active and oxide layers begin forming immediately upon exposure to the atmosphere. Ammonium chloride with zinc made as flux in aqueous solution is applied to the steel to reduce any oxides that are formed and/or inhibit them from forming altogether. This allows the molten zinc in the proceeding galvanizing step to maximally adhere to and alloy with the surface of the steel.
Ammonium chloride is sold in block or tablet form (slab, brick, lata, wadi, danda) at hardware stores for use in cleaning the tip of a soldering iron, and it can also be included in solder as flux.
If you have any questions about our products and services, please contact us at +91 93775 12003 , modheshwarichemicals@yahoo.co.in or complete the online form.